SAFETY
See the findings on XCOPRI safety
MOST COMMON XCOPRI ADVERSE REACTIONS (≥10% FOR XCOPRI AND GREATER THAN PLACEBO) FOR PATIENTS TAKING 200 MG/DAY OF XCOPRI (RECOMMENDED MAINTENANCE DOSAGE)1*
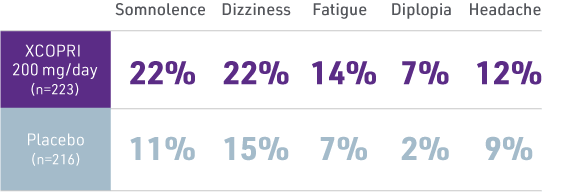
XCOPRI 100 mg/day (n=108)
and XCOPRI 400 mg/day (n=111);
somnolence, 19% and 37%;
dizziness, 18% and 33%;
fatigue, 12% and 24%;
diplopia, 6% and 15%;
headache 10% and 10%.
No dosage changes to concomitant anti-seizure medications (ASMs) were allowed during the double‑blind clinical trials2,3
This is not a full list of safety information.
For more information, see Full Prescribing Information.
Read more about XCOPRI’s efficacy
See Efficacy