MECHANISM OF ACTION
XCOPRI reduces neuronal excitability through a dual mechanism of action*
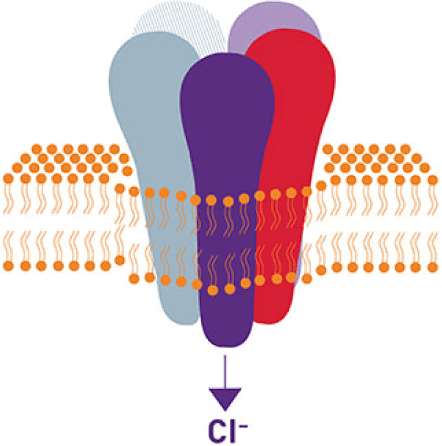
Positive Allosteric Modulator of the GABAA Receptor
Potentiates GABAA-receptor inhibition by enhancing the inward movement of chloride ions.1-3
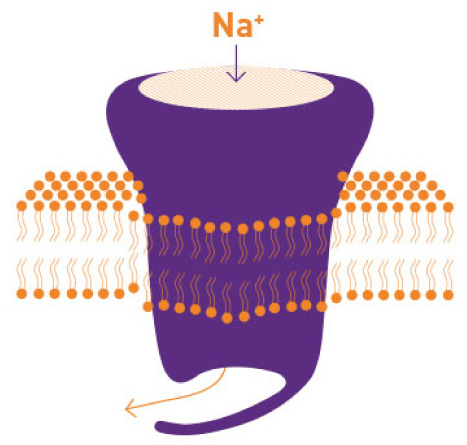
Inhibits Persistent Sodium Current
Reduces repetitive neuronal firing by inhibiting the persistent component of the inward sodium current.1,4
XCOPRI in action
Explore the efficacy data for XCOPRI and see how it may impact your patients’ lives
See Efficacy