SAFETY
The safety profile of XCOPRI offers more than a decade of clinical trial and real-world
experience, including long-term retention data.
Actor portrayals.


The safety profile of XCOPRI offers more than a decade of clinical trial and real-world
experience, including long-term retention data.
10+ years of clinical trial and real-world experience
Well-studied safety with over 220,000 patients prescribed worldwide*
Most common adverse reactions observed in patients during XCOPRI (cenobamate tablets) CV clinical trials include1†:
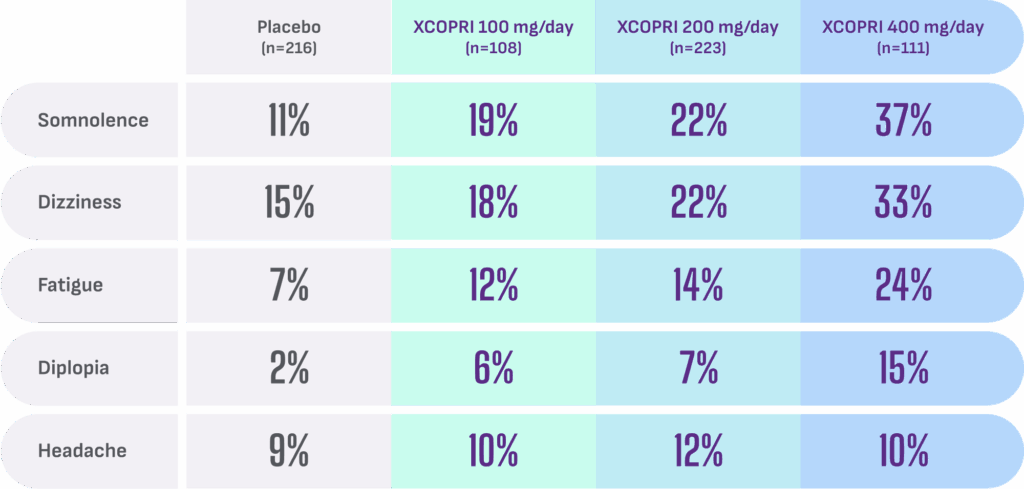
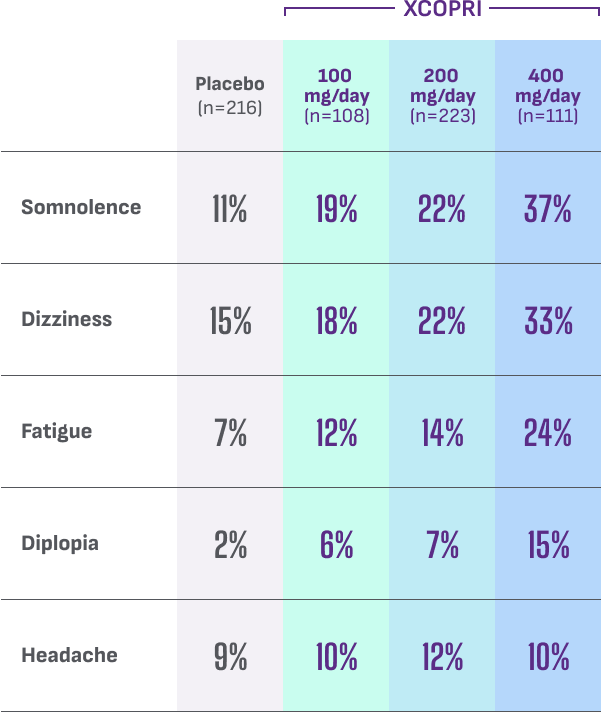
- Discontinuation rates due to adverse reactions were 11% (100 mg), 9% (200 mg), and 21% (400 mg) for patients on XCOPRI compared with 4% for patients on placebo1
- No dosage changes to concomitant anti-seizure medications (ASMs) were allowed during the double-blind clinical trials2,3
- XCOPRI makes the concentration of some drugs increase. Consider decreasing the dosage of clobazam, phenytoin, and phenobarbital early in XCOPRI titration1
XCOPRI was studied across 2 multicenter, randomized, double-blind, placebo-controlled trials in adult patients with partial-onset seizures with or without secondary generalization.1
1-3
concomitant ASMs were not adequately controlling patients at baseline1‡
PLACEBO
Patients in the placebo arm remained on their current ASM2,3
of patients were taking 2 or more concomitant ASMs1
24YEARS
was the approximate duration of epilepsy for patients in both studies1
~9SEIZURES
per 28 days was the median baseline seizure frequency for patients in both studies1
Cognition and weight with XCOPRI
Based on a post hoc analysis of pooled populations across phase 2 and phase 3 studies
Rates of cognitive TEAEs observed with XCOPRI were 2.3% vs 0.9% with placebo4§
Limitations: Cognitive symptoms were evaluated using non-standardized measures including spontaneous patient self-reporting, which may have led to underreporting of mild or intermittent symptoms. Baseline cognitive comorbidities were assessed retrospectively and could not be directly compared with TEAEs. Potential confounding effects from concomitant ASMs further limit interpretation of cognitive findings.
Low rates of weight change reported with XCOPRI in pivotal trials1
XCOPRI (3/442) vs placebo (0/216)
TEAEs=treatment-emergent adverse events.

Long-term retention rates5
Pooled data from the clinical development program:
At 5 years, the majority of patients remained on treatment
About the analysis:
These retention data are based on Kaplan-Meier estimates of time to discontinuation of open-label XCOPRI in the pooled population from 3 clinical studies. Conclusions of long-term efficacy and safety should not be drawn based on these data.
80% of patients were on 2-3 concomitant ASMs at the start of open-label XCOPRI treatment. In this pooled population, the median duration of XCOPRI exposure was 2.8 years, with a median XCOPRI modal dose of 200 mg/day.

“Adjustments in the dosage of medications in patients taking multiple drugs can help optimize efficacy while minimizing side effects.”
– Pavel Klein, MB BChir, FAAN, FAES
Watch expert videoDosing
Convenient once-daily dosing with a personalized treatment approach for your patients
Discover dosingAccess
XCOPRI is widely accessible with low copays. Discover support available to get started
Learn about access